Center for Communications, Health and the Environment

RISK, REGULATION AND REWARD:
GE Crops Are Here to Stay, But Do Their Purported Benefits Outweigh Their Potential Risks?
by Gregory Jaffe, Director, Biotechnology Project, Center for Science in the Public Interest, Washington, D.C.
Last year, 158 million acres of genetically engineered (GE) crops were grown across the United States, according to the International Service for the Acquisition of Agri-biotech Applications (ISAAA), a nonprofit that monitors these crops. Among the GE varieties harvested were 85 percent of all U.S. corn, 91 percent of all U.S. soybeans, and 88 percent of all U.S. upland cotton, reported the U.S. Department of Agriculture’s National Agricultural Statistics Service. Most of the field corn and soybeans were used for animal feed and industrial purposes (such as corn for ethanol production). Some of them were also used to produce popular food ingredients such as corn oil, soybean oil, high fructose corn syrup and corn meal, ensuring that the majority of Americans currently consume everyday foods with ingredients made from engineered crops.
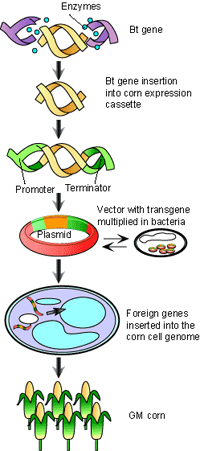 |
| Figure 1. General schematic of GM crop production (Source: The Science Creative Quarterly, Issue 4, 2009) |
Since their debut in the 1990s, GE, or biotech, crops have become part of mainstream agriculture in the United States, and many other developed and developing countries. Their advent has not been without controversy and concern for human health, and critics, as well as devotees, are plentiful. To date, however, the risks raised have not materialized. And while not all the merits touted by the developers have emerged either, significant benefits have been documented, and adoption of GE crops is on the rise worldwide.
A Snapshot of Genetic Engineering
Genetic engineering in agriculture involves taking a gene from one species and introducing it into a cell of another species to produce a desired trait or product in the resulting organism and its progeny. After scientists introduce the foreign gene into the plant cell in the laboratory, farmers sow the engineered seeds, and the resulting harvest is used to feed (or clothe) humans or animals, or to produce food ingredients, such as GE corn for corn flakes or oil from engineered soybeans for salad dressing.
U.S. farmers began growing engineered crops in the mid-1990s when herbicide-tolerant soybeans and delayed-ripening tomatoes were commercially released. Engineered varieties of corn, cotton, canola, potato, squash and papaya that were herbicide-tolerant, virus-resistant or produced their own pesticides followed a few years later. These products helped to solve farm production issues by enabling farmers to increase yields and/or reduce costs by lessening chemical inputs and labor expenses, for example. Adoption of engineered corn and cotton varieties by U.S. farmers increased tremendously between 2003 and 2008, when developers commercialized strains with multiple “stacked” genes – varieties that provided both herbicide tolerance and pest protection, or produced multiple pesticides to combat a variety of insect problems.
The Cultivation of Biotech Crops
Given recent trends, over the course of this year, American and global production of GE crops will continue to grow. Meanwhile, the ISAAA reports that, worldwide in 2009, 14 million farmers – 90 percent of them small, resource-poor farmers from 16 developing nations – planted 330 million acres of GE crops in 25 countries, up 7 percent from 2008 in both number of farmers and acreage. Today, the United States remains the top producer of engineered crops, followed by Brazil, Argentina, India, Canada and China.
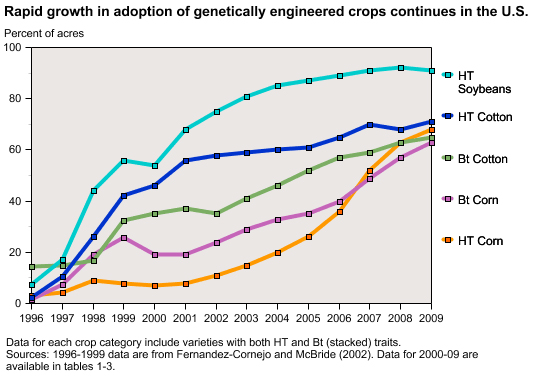 |
| Source: USDA Economic Research Review |
Among developing countries, India and China have been the most significant adopters, with approximately 12.6 million mostly smallholder farmers growing almost 30 million acres of insect-protected cotton and other GE varieties. In Europe, on the other hand, GE crops are extremely controversial. Consumer and environmental organizations have questioned their safety and do not want to support U.S. multinational corporations that produce the engineered seeds. In addition, the introduction of GE crops occurred during a time when the region faced a number of food-safety problems. For these and others reasons, today only six European countries grow biotech crops (Germany discontinued GE planting in 2008), and those that do, such as Spain, Portugal and the Czech Republic, grow small amounts; in addition, GE products are not found in most European supermarkets.
Adoption of first-generation GE crops has been driven by the economic benefits that accrue to farmers. In the United States, for example, farmers growing conventional cotton use significant amounts of pesticides to reduce insect damage; but farmers who adopt GE cotton reduce the number of pesticide applications by as much as 50 percent, resulting in a total national decrease of almost 3 million pounds of such pesticide per year, according to the National Center for Food and Agricultural Policy. Meanwhile, adoption of herbicide-tolerant soybeans by U.S. farmers has substituted an herbicide believed to be more environmentally friendly than that used on non-engineered seed; it also has increased farmers’ use of environmentally beneficial no-till farming and reduced their farm management time, enabling them to increase off-farm income, detailed the 2006 edition of "Agricultural Resources and Environmental Indicators," a publication of the U.S. Department of Agriculture’s Economic Research Services.
Controversy and Risk Assessment
 |
| Source: www.eyeondna.com |
While current and future GE crops may yield significant benefits, they are also controversial because genetic engineering involves potential risks. To date, no commercial GE crop has presented any food-safety risk or resulted in a documented human health effect; nevertheless, each new product must undergo an individual food-safety risk assessment. Developers need to ensure – and the government must verify – that the introduced gene does not produce an allergen or toxin and that, by engineering the plant, there is neither reduction in its nutritional profile nor the migration of harmful substances normally produced elsewhere in the plant to any edible portions. Extensive laboratory testing needs to be done before any biotech crop enters the food supply. No consumer wants to, nor should, eat a product from a GE crop unless an independent regulatory authority has verified its safety.
Environmental risk assessment and management of GE crops is also necessary to ensure that biotech crops don’t negatively impact our environment more than current conventional agriculture. Some environmental questions and concerns surrounding GE crops include:
- Can engineered plants transfer their introduced genes to wild relatives, and what effect could that have (e.g., weediness)?
- Can GE crops transfer their genes to native land races and impact biodiversity?
- How might these newly produced biotech substances impact non-target organisms (such as monarch butterflies, grasshoppers and deer)?
- Will pests evolve resistance to the pesticides incorporated in GE crops and return farmers to the chemical pesticide treadmill?
(For a more detailed discussion of the potential environmental risks of GE crops, click here.)
The most significant risks from GE crops to date, however, have been socio-economic and commercial. When Starlink corn, a GE variety only approved for animal feed, got into the U.S. food supply, it resulted in a more than $1 billion recall of food products. More recently, when rice planted in the United States was found to be contaminated with small amounts of an unapproved, albeit safe, biotech variety, U.S. products were not recalled domestically because the regulatory agencies determined there was no food safety risk; however, trade with countries such as Japan was affected, greatly impacting American rice farmers. In addition, the growing of GE crops that have been approved in the United States but not in trading partner countries in Europe, for example, has reduced U.S. exports of some commodities, and forced the channeling of exports – at lower prices and volumes – to specific domestic and international markets.
Regulatory Systems, Situations and Shortcomings
To address the potential risks of GE products, the United States, the European Union and many developed nations such as Japan and Canada have established and maintain functioning biosafety regulatory systems. These systems review and usually are required to approve GE crops before they are planted, harvested and enter the food supply. They employ existing laws and/or newly established ones, and vary in their scrutiny of GE products. Other countries, many of them developing ones such as Vietnam, Malawi and Ghana, do not yet have functional biosafety regulatory systems, but will likely have them soon. Meanwhile, more than 157 countries worldwide, including many African and Asian nations, are parties to the Cartagena Biosafety Protocol, an international trade agreement that requires the establishment of a national biosafety regulatory system to assess and manage potential risks of GE organisms. Noticeably, principal GE producers Argentina, Canada and the United States have not ratified the agreement to date, although America “participated in the negotiation of the text and the subsequent preparations for entry into force under the Intergovernmental Committee on the Cartagena Protocol” in 2003 and has been an observer at the meetings of the parties held after the Protocol came into effect, according to the U.S. Department of Agriculture (USDA) Website.
The United States does, however, adhere to and maintain a federal regulatory system for GE products that, while perhaps not as stringent as other nations’, tends to be more transparent and participatory. The U.S. biosafety regulatory system started in 1986 with the “Coordinated Framework for Regulation of Biotechnology.” This document primarily focuses on three agencies – the Environmental Protection Agency (EPA), USDA, and the Food and Drug Administration (FDA) – and was a creative attempt to regulate an emerging technology using existing laws. Under the framework, EPA uses the Federal Insecticide, Fungicide, and Rodenticide Act to regulate engineered crops that incorporate pesticides to ensure that these pesticides are environmentally safe and don’t leave residues that are harmful to humans on the food. EPA has registered engineered crops under its authority and often imposes grower obligations to safeguard the environment and protect the longevity of the technology. USDA regulates the release of any engineered plant under the Plant Protection Act to ensure that cultivating GE crops doesn’t result in a plant pest risk or other harm to agricultural interests. For experimental crops, this involves imposing planting conditions that prevent cross-pollination and persistence of the engineered crop in the environment after a field trial has been completed. The FDA applies the Federal Food, Drug, and Cosmetic Act to ensure that foods made from biotech crops are safe for human and animal consumption.
 |
| Biotech crops are subject to regulation, but a reevaluation of the existing framework may be in order (Source: www.rebuild-from-depression.com/blog) |
While instituting a case-by-case review by two and sometimes three of these regulatory agencies, the Coordinated Framework has, however, resulted in gaps and ambiguities in product regulation, as agencies apply standards and procedures not designed to address GE products directly. More specifically, the framework lacks adequate oversight to safeguard America’s food supply, protect its environment and encourage widespread consumer acceptance of safe products. For example, FDA’s regulation of biotech crops does not require mandatory pre-market approval, but instead involves a voluntary consultation process. While such regulation may be welcomed by developers, it puts the burden on FDA to find a product potentially unsafe before it can prevent its introduction into the food supply – a backward, impractical and potentially dangerous arrangement. It also means that the public is relying on the industry’s self-interested safety determination when it comes to GE products, instead of an independent safety assessment. Meanwhile, USDA ensures that engineered crops are not plant pests, but it does not address the broad environmental impact of growing GE crops across millions of U.S. acres. The agency also has had difficulty ensuring that experimental crops don’t persist in the environment or enter the food supply after the growing season is over.
In the February 12, 2010 issue of Science, Nina Fedoroff of the U.S. State Department and 14 other authors contend that “...the benefits of biotechnology have not been realized for the vast majority of food crops” as a result of a complex, costly and time-intensive regulatory apparatus and the virtual exclusion of public-sector research, and molecular knowledge and techniques to improve harvests. They argue for a reevaluation of the existing regulatory framework along with an authoritative assessment of data on genetically modified, or engineered, crop safety that encompasses “protein safety, gene stability, acute toxicity, composition, nutritional value, allergenicity, gene flow, and effects on nontarget organisms.” They also recommend that the USDA conduct safety testing of GE crops developed in the public sector. Such measures would, they contend, level the playing field and set a welcome global precedent.
The Future of GE Crops and Products
In the meantime, GE crops continue to be consumed without incident. Over the past decade, they have increased agricultural productivity and farm incomes, and delivered environmental and health benefits, including the decreased use of pesticides and an increase in no-till farming. Under these conditions, over the next decade, biotechnology companies will continue commercializing additional GE herbicide-tolerant and pesticide-producing varieties for commodity crops.
The next 10 years could also see second-generation biotech products, which will be much more diverse – and possibly more controversial. Second-generation products might include complex input traits helpful to farmers such as salt tolerance, nutritionally enhanced products beneficial to consumers, and plants and animals “designed” to produce pharmaceuticals. New engineered crops could include drought-tolerant corn and cotton, and corn and soybeans that produce healthier oils (including omega-3 compounds).
The coming years could also see the commercialization of GE crops developed by public institutions and private companies, and products that are specifically adapted to developing-country farmers and consumers.
Future actions of developers and regulators will determine, however, whether the industry continues to mature and advance new products around the world in a safe manner. And the U.S. government, in particular, needs to revise its biosafety regulatory system to ensure that GE products are adequately regulated, and are truly safe for both humans and the environment. If a strong, but non-stifling, regulatory system can be implemented, investment in this promising technology will advance, consumers can have confidence in the new products, and benefits will continue to be realized.
Read More:
Lead Article: Risk, Regulation, and Reward
Spotlight Article: Biotech Crops Gain Global Foothold
CECHE News: U.S. Food Safety Reform Takes Off
Also Noted: What Risks Do Genetically Engineered Crops Pose to the Environment?

Dr. Sushma Palmer, Program Director
Valeska Stupak, Editor & Design Consultant
Shiraz Mahyera, Systems Manager
Daniel Hollingsworth, Website Consultant